
Nitrogen trifluoride Alchetron, The Free Social Encyclopedia
This chemistry video will show you how to draw the Lewis structure and determine the molecular geometry for nitrogen trifluoride (NF3).

How to Calculate the Formal Charges for NF3 (Nitrogen trifluoride
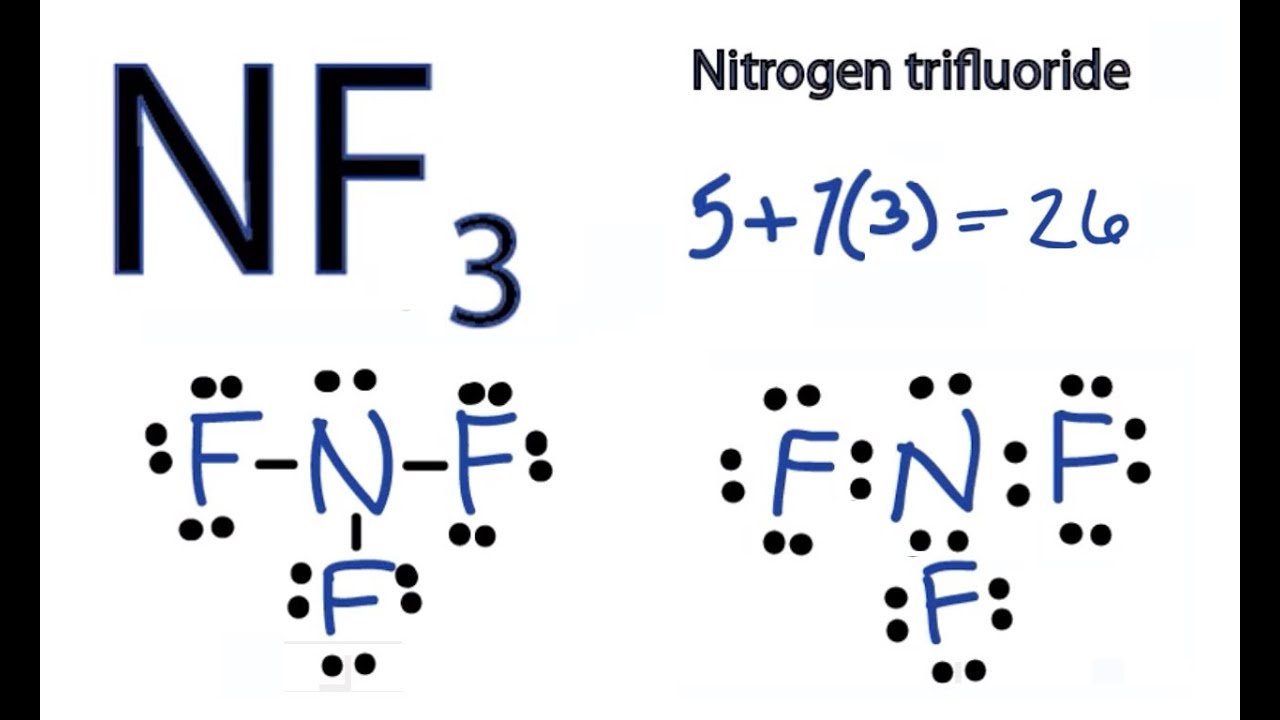
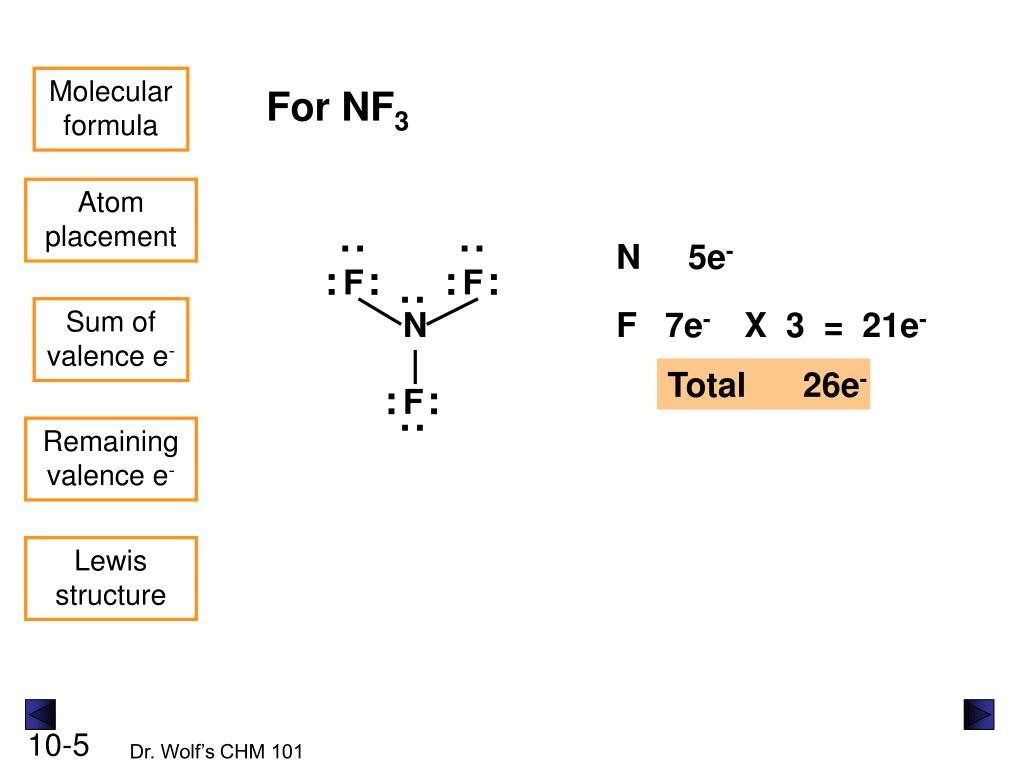
In the lewis structure of Nitrogen trifluoride (NF 3), there are three N-F bonds and one lone pair on nitrogen atom. Each fluorine atom has three lone pairs. Lewis structure of NF 3 can be drawn by starting from valence electrons of nitrogen and fluorine atoms in several steps. Each step of drawing the lewis structure of NF 3 is explained in.

NF3 Molecular Geometry,Shape and Bond Angles (Nitrogen Trifluoride
The trigonal pyramidal molecular geometry of NF3 is consistent with this deviation from the predicted bond angle. Thus, the observed bond angle for NF3 is around 102 degrees, slightly smaller than the predicted bond angle. The presence of the lone pair of electrons is the reason for this slight difference in bond angle.

Nf3 Molecular Geometry Bond Angles
The total valence electron available for the NF3 lewis structure is 26. Hybridization of NF3 is Sp³. NF3 is polar in nature. The molecular geometry or shape of NF3 is a trigonal pyramid and its electron geometry is tetrahedral. NF3 lewis dot structure contains 1 lone pair and 3 bonded pairs.

NF3 Molecular Geometry Science Education and Tutorials
A step-by-step explanation of how to draw the NF3 Lewis Dot Structure (Nitrogen trifluoride).For the NF3 structure use the periodic table to find the total n.

Stable configuration of NF3 molecule after optimization at
Molecular geometry describes the actual shape of the molecule and is determined by the a. Part A What is the molecular geometry of NF3? Enter the molecular geometry of the molecule. View Available Hint (s) Submit v Part B What is the molecular geometry of CS2?

Which out of NH3 and NF3 has a higher dipole moment and why?
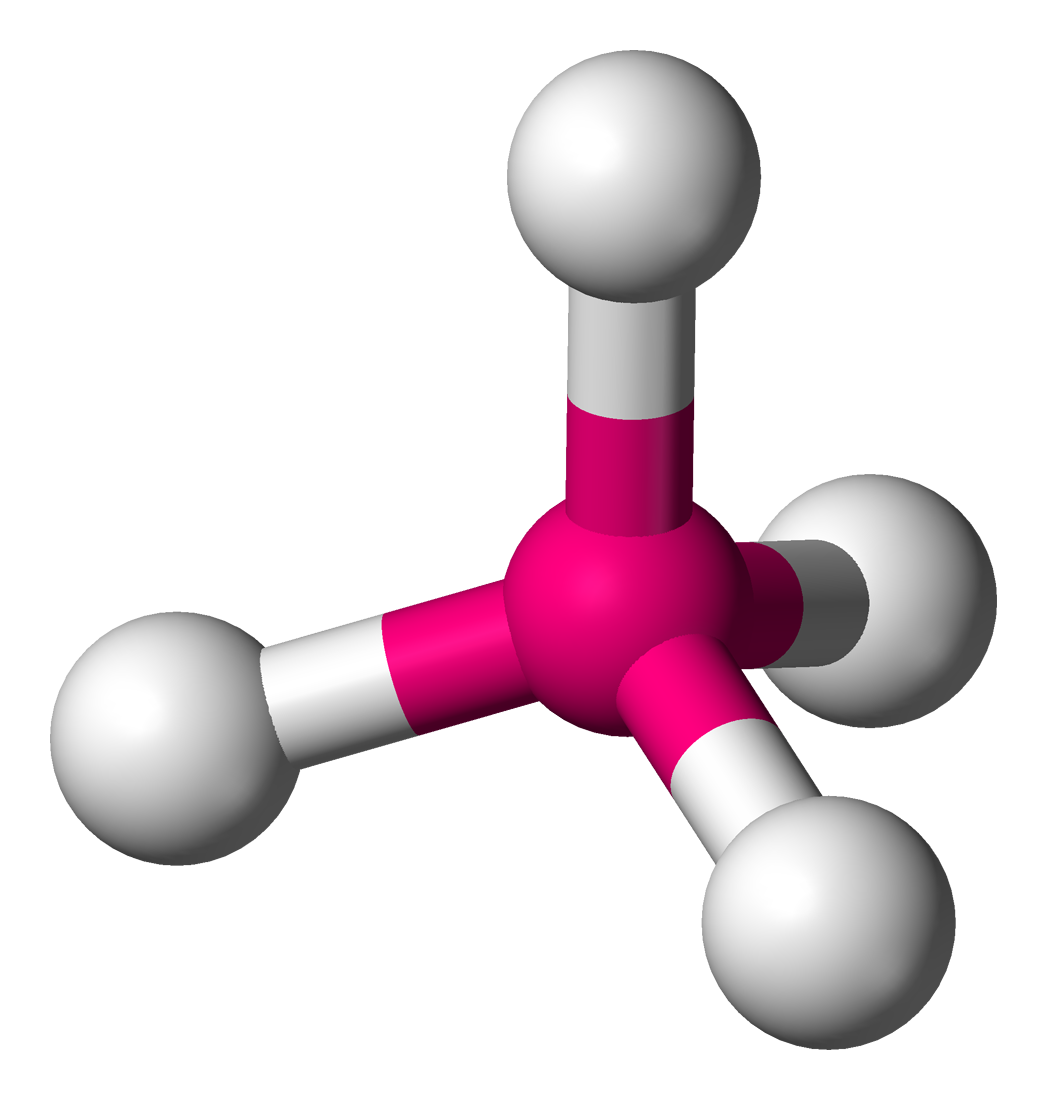
Nitrogen trifluoride (NF3) lewis structure contains three sigma bonds and one lone pair around nitrogen atom. Therefore, there are total of four electrons regions around nitrogen atom. So, hybridization of center atom, nitrogen is sp3. Because there are four electrons regions, geometry is tetrahedral and shape is trigonal pyramidal.
What is the tetrahedral molecular shape of NF3? proquestyamaha.web
NF3 lewis structure angle As shown in the above figure, the bond angle is about 102.5 o in NF 3 as the molecular geometry is trigonal pyramidal and has a lone pair due to which the standard tetrahedral angle of 109 o has deviated and decreased to 102.5 o.. NF3 uses. Nitrogen trifluoride is primarily used for manufacturing microelectronics such as LCDs and thin-film solar cells, as.

Nf3 Molecular Geometry Bond Angles
Nitrogen trifluoride (NF 3) is an inorganic, colorless, non-flammable, toxic gas with a slightly musty odor. It finds increasing use within the manufacturing of flat-panel displays, photovoltaics, LEDs and other microelectronics. Nitrogen trifluoride is also an extremely strong and long-lived greenhouse gas.Its atmospheric burden exceeded 2 parts per trillion during 2019 and has doubled every.

How to draw NF3 Lewis Structure? 4
The molecular geometry of NF3 is trigonal pyramidal, which means the three fluorine atoms are arranged around the central nitrogen atom with a slight bent shape. This geometry results in an unequal distribution of charge, and the molecule has a net dipole moment, making it polar. The partial negative charges are centered on the fluorine atoms.
draw lewis structures for the following molecules nf3
Nitrogen trifluoride appears as a colourless gas with a mouldy odour. Very toxic by inhalation. In this video, we will look at the molecular geometry of an N.

Nf3 Molecular Geometry Bond Angles
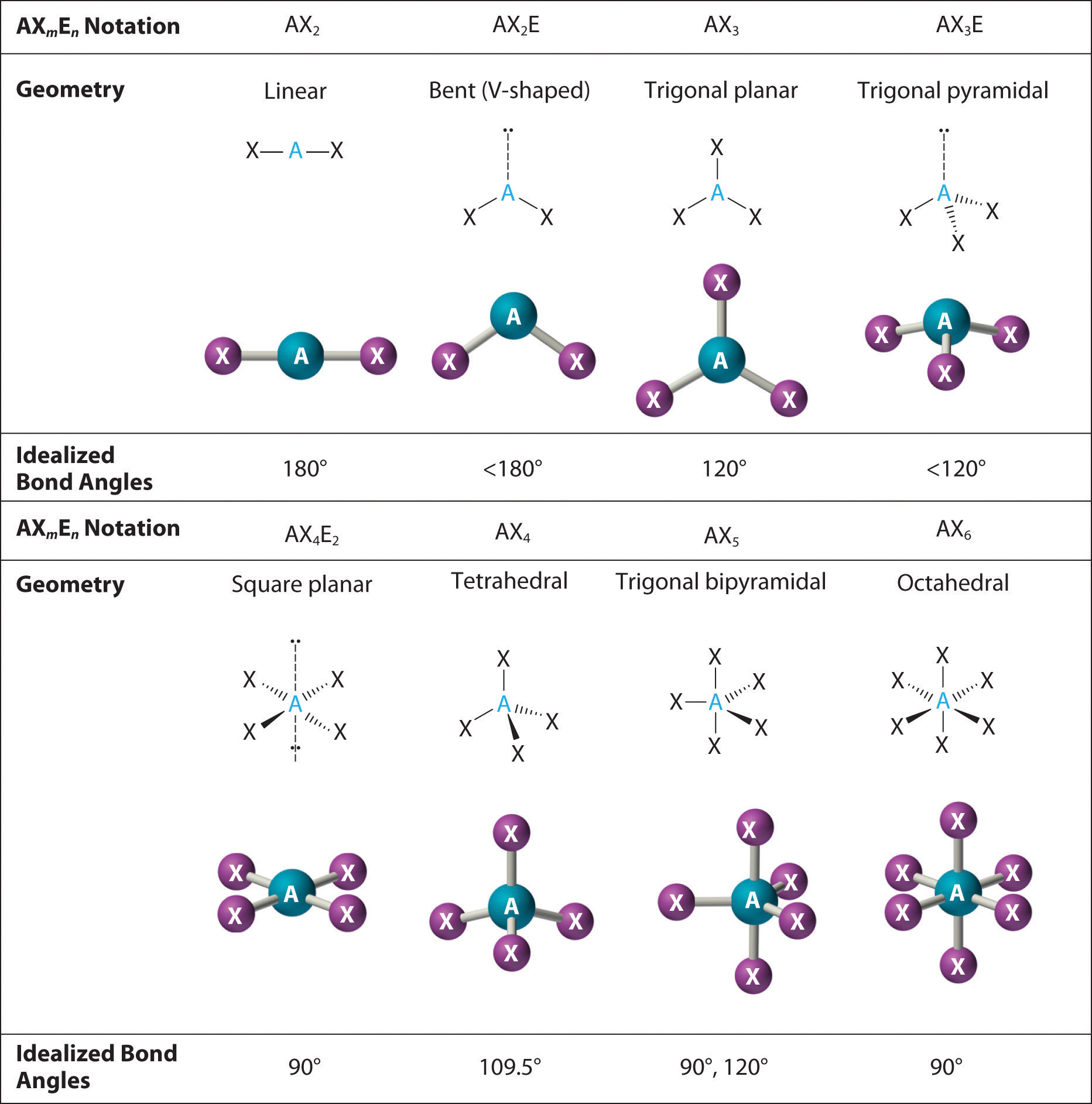
Figure 5.2.9 5.2. 9: (a) H2O has four regions of electron density around the central atom, so it has a tetrahedral electron-pair geometry. (b) Two of the electron regions are lone pairs, so the molecular structure is bent. Exercise 5.2.3 5.2. 3. The hydronium ion, H 3 O +, forms when acids are dissolved in water.
9.7 The Shapes of Molecules Chemistry LibreTexts
A quick explanation of the molecular geometry of NF3 including a description of the NF3 bond angles.Looking at the NF3 Lewis structure we can see that there.
Nf3 Lewis Structure Free Hot Nude Porn Pic Gallery
A three-step approach for drawing the NF3 molecular can be used. The first step is to sketch the molecular geometry of the NF3 molecule, to calculate the lone pairs of the electron in the central Nitrogen atom; the second step is to calculate the NF3 hybridization, and the third step is to give perfect notation for the NF3 molecular geometry.
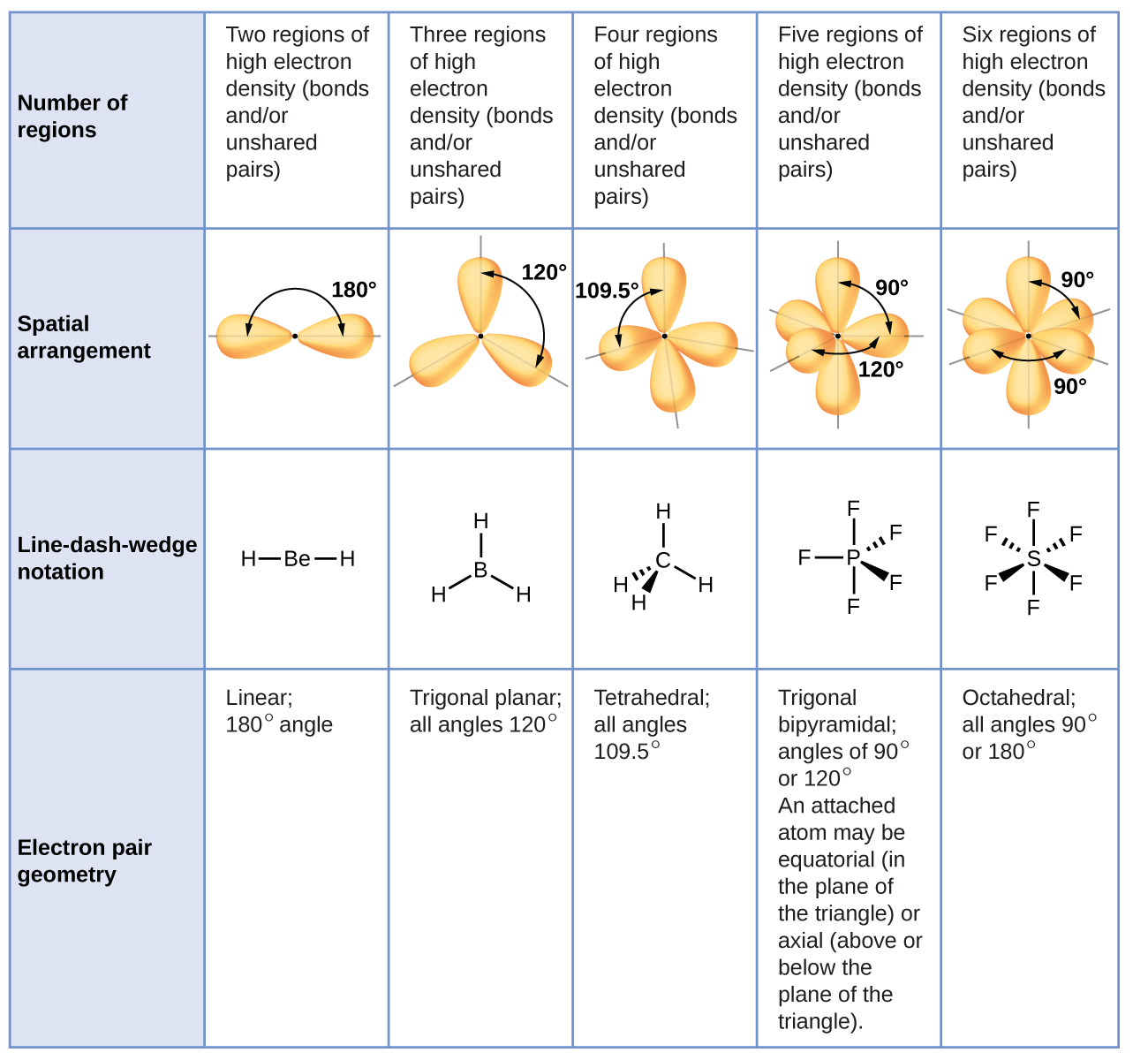
5.2 Molecular Shape Chemistry LibreTexts
In this blog post, we will explore the Lewis structure for NF3, its bond angle, and whether it is polar or nonpolar. We will also answer questions like how many bonds NF3 has and dive into its molecular geometry. So, let's dive in and unravel the mysteries behind NF3! Lewis Structure for NF3: A Molecular Dance of Nitrogen and Fluorine

NF3 Draw the molecule by placing atoms on the grid and connecting them
The molecular shape of NF3 is_____. The N-F bond is _____. (polar or non polar) The F-N-F angle is approximately_____. A NF3 molecule is_____.(polar or non polar) This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer See Answer.